This site uses cookies to enhance your browsing experience. By continuing navigation, you agree to the use of cookies. For more information about our use of cookies, see our Privacy Policy.
This site uses cookies to enhance your browsing experience. By continuing navigation, you agree to the use of cookies. For more information about our use of cookies, see our Privacy Policy.
Advancing the Treatment of Heart Failure. Together.
Successful heart failure (HF) management requires a combination of lifestyle changes and frequent medical therapy adjustments. With the FDA-approved Cordella PA Sensor and HF System, we make it easier for patients with NYHA class III heart failure, caregivers, and clinicians.
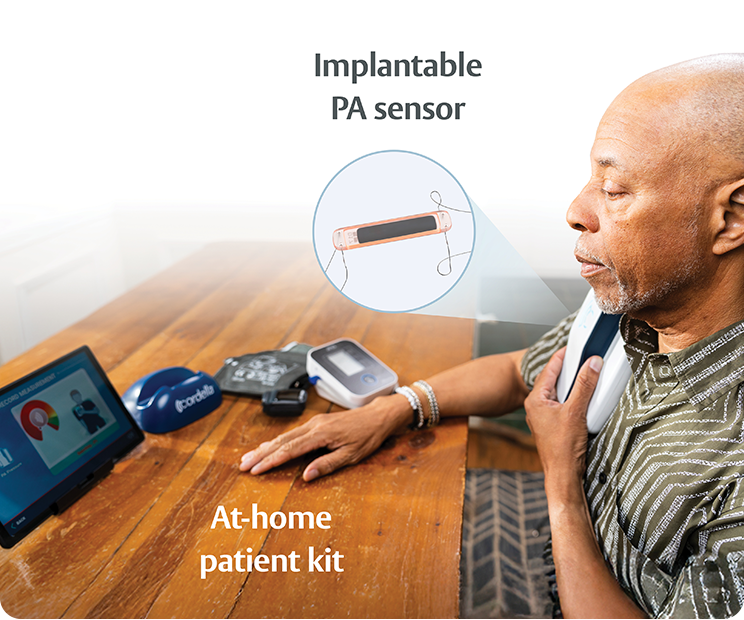
Guides HF management that may reduce congestion & improve outcomes1
Enables remote HF medication optimization1
Encourages patient self-awareness & sustainable lifestyle decisions
Meet Tom
Meet David
THE EVIDENCE IS IN FOR NYHA CLASS III PATIENTS
Markedly low 12-month HF Hospitalization / all-cause mortality rate (0.36)
Significant improvement in key quality of life metrics and functional capacity (KCCQ, 6-minute walk test, and NT-proBNP)*

Reduction in HF hospitalization rate before and after Cordella use at
12 months
*KCCQ (+5.7 points, p<0.0001), 6-minute walk text (+35 m, p=0.0004), NT-proBNP (-268 pg/mL, p=0.006)
DSRC: Device or System-Related Complications; KCCQ: Kansas City Cardiomyopathy Questionnaire

“[Cordella] is an amazing device. It’s a tool that everyone should have access to. It is the future of medicine in our minds.”
–Anita, Caregiver of a Cordella patient

References